|
|
Environmental Consultants |
There are few manufacturing industries to-day which can dispense with the services of the chemist, and the vast field covered by the chemical industry of to-day is the natural result of co-operation between the chemist and his fellow scientists, or between the chemist and the engineer and manufacturer. Quite truthfully the chemical industry has become the foundation on which not only British industry as a whole but also modern world-industry is erected. Not only has chemistry become the servant of the older industries but in many cases the powerful rival, and in some cases the master. The synthetic products produced by the combined ingenuity of engineer and chemist not only serve to meet demands created by a high standard of living, but come definitely into comp etition with substances of natural origin, or even the earlier products of the chemists themselves. Thus, synthetic nitrates are more than adequate competitors for the favours of the agriculturalist requiring nitrates for fertilizing. The quantity of artificial silk and chemical fibres used in the world now exceeds the quantity of natural silk. The last two or three decades have seen chromium plating replace nickel plating just as nickel plated goods replaced brass and bronze of earlier periods. Similarly, plastics have largely replaced metals —especially brass—and also wood, glass, leather and textiles for many purposes. In the near future oil produced by the hydrog eneration of coal may possibly replace natural mineral oil when supplies of the latter begin to diminish.
The heavy chemical industry is in many
respects the chemical industry proper, and embraces the manufacture of those
commodities which are required in large quantity and have a variety of uses. The
birth of the whole chemical industry took place little more than 200 years ago,
and indeed, at the close of the eighteenth century there was scarcely a chemical
industry in this or any other country —apart from the manufacture of gunpowder,
a few acids in small quantities, and a few drugs.1 One of the first chemicals
produced in quantity was sulphuric acid, manufactured in England as early as
1720. The discovery of the value of chlorine as a bleaching agent in 1785 opened
up a new avenue of manufacture, and towards the close of the eighteenth century
the discovery of bleaching powder, or chloride of lime, by Charles Tennant, led
in 1797 to the foundation of the chemical works at St. Rollox in Glasgow. Within
a few years the manufacture of bleaching powder by passing chlorine over dried
powdered lime was an important industry in many parts of the country. In 1790
the French Government awarded a prize to Nicholas Leblanc for a method of making
soda, since France, owing to wars, had found it difficult to obtain a constant
supply of this commodity. His method consisted of treating common salt with
sulphuric acid, thus making sodium sulphate and liberating hydrochloric acid
gas, then roasting the sodium sulphate with limestone and charcoal or coal and
obtaining in this way sodium carbonate and calcium sulphide. The growth of the
Leblanc soda process in this country was slow, but it is not difficult to see
that salt is the primary raw material in the manufacture of soda and alkalis.
Thus, the manufacture of soda, sooner or later, gravitated to the one great salt
field of this country then known—the Cheshire salt field. It was not long before
the factory of a soda manufacturer tended to become larger and more complicated.
| He not only made soda, using common salt, sulphuric acid, and other raw materials, but he started to make his own sulphuric acid by burning sulphur or pyrites. He produced large quantities of hydrochloric acid which at first were allowed to escape into the atmosphere with terrible results on areas around the chemical works. So it is easy to see how he became a manufacturer of acids, more especially of nitric acid, which is required in the manufacture of sulphuric. Then followed the various salts of sodium, copper, and iron. It was again a common development to use the chlorine recovered to make bleaching powder, and more and more efforts were made to utilise what had at first been waste products. Thus, at first, for every ton of soda made nearly two tons of alkaline waste were produced, |
|
an evil-smelling mass containing practically all the sulphur from the sulphuric acid used; and, although by a process introduced in 1861 a third of the sulphur was recovered, it was not until 1882 that waste was really eliminated. The elimination of waste was more or less forced on the chemical manufacturers by the Alkali Act of 1863, which provided for close inspection, and for heavy penalties against emitting obnoxious fumes into the atmosphere. By 1890 the British heavy chemical industry was thus in the hands of forty or fifty firms whose works were situated principally on the salt-field of Cheshire and the neighbouring parts of the mid-Mersey region of Lancashire. As soda manufacturers they were suffering from the severe competition of what is known as the ammonia-soda process for the manufacture of soda. The chemistry of the process involved in the mixing of salt, ammonia, carbon dioxide, and water to form bicarbonate of soda and ammonium chloride is very simple, but it was not until 1856—1866 that the Solvays of Belgium were successful in producing soda in quantity by this process. It was they who, in 1873, granted a licence to Brunner, Mond & Co., a combination of Brunner the administrator and Mond the energetic young chemist, to manufacture in Great Britain. In 1890 the British heavy chemical firms were forced into combination and formed the United Alkali Co. of Liverpool. Although the object of the combination was to facilitate, by lowering costs, the continuance of the manufacture of soda by the Leblanc process, they were forced in turn to take up the ammonia-soda process. Some idea of the progress may be judged by saying that in 1863 the world’s production of soda was 300,000 tons and the price about £13 per ton. In 1902 out of an annual production of 1,800,000 tons, 1,650,000 were made by the Solvay process, and the selling price was only about £4 per ton.
To go back somewhat, the close connection
between the heavy chemical industry and many other industries was apparent
early. With the Industrial Revolution the growth of the textile industry meant
an increase in demand for acids, alkalis, soaps, and chemicals of all kinds. The
increased use of soap all over the world meant increased demand for the alkalis
used in its manufacture. Thus, there was a dual marriage of the chemical and the
textile industries, the chemical and the soap-making industries. The
ramifications of the chemical industry became increasingly apparent towards the
latter half of the nineteenth century. The year 1887 saw the patenting of the
famous Melver-Forrest Cyanide process for extracting gold and silver from
refractory ores and from “tailings” —a process which has revolutionised gold
mining, for there are few mines existing to-day which can be operated properly
without a cyanide annexe to the mill. This has meant the extensive manuf acture
of cyanide in this country. Then the early experiments of Mr. J. B. (later Sir
John) Lawes, who succeeded to the Rothamsted estate in Hertfordshire, led to the
appreciation of various artificial manures, and in 1843 Mr. Lawes established
large works in the neighbourhood of London for the manufacture of superphosphate
of lime.
The outbreak of war in 1914 found the chemical
manufacturers of this country quite unprepared. There were government factories
well equipped for the manufacture of explosives, but they were only on a small
scale. Firms, such as Messrs. Nobel, had up-to-date works, but again on a small
scale. Apart from some of the larger firms such as Brunner, Mond & Co. the
smaller alkali manufacturers had works which were far from up-to-date. At the
beginning of’ the war most of our shells were filled with
picric acid as an
explosive, for T.N.T. or trinitrotoluene had only just been adopted. The first
government factory for its manufacture was not commenced until February,
1915,
although exactly three months later the first ton of
T.N.T. was produced
and packed for delivery and the factory was very soon producing to capacity. Of
necessity the explosive firms in particular and the chemical firms in general
had during the war period to work together and to pooi their knowledge. The way
was thus paved for the formation of the combine in November, 1918, and limestone
of Explosive Trades, Ltd., afterwards known as Nobel Industries, Ltd., the chief
establishment of which was at Ardeer on the Ayrshire coast. There thus came to
be four great firms concerned with the chemical industries of this country:
Brunner, Mond & Co., Nobel Industries, Ltd., the United Alkali Co., and British
Dyestuffs Corporation, Ltd. The amalgamation of these came naturally in 1926
with the formation of Imperial Chemical Industries, Ltd., the British combine
with a capital of £65,000,000 sterling.
Geographical
Distribution of Chemical Industry
Geographical factors in the distribution of
the various branches of the chemical industry are fairly obvious, and many of
the early locational influences—including sources of salt and fuel, and inland
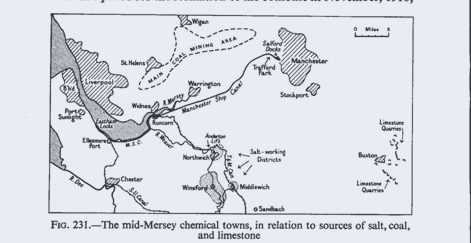
water transport—continue to be important. The greatest concent ration of the
industry is in the mid-Mersey region and the Cheshire saltfield.1 Here on the
saltfield is the Headquarters of the J.C.I. Alkali Division, at Winnington,
Northwich (the former Brunner, Mond Works, established in 1873 alongside the
River Weaver). Some
50
per cent. of the insured population of Northwich is
employed in the chemical industry. Also on the saltfield are Winsford, where
45
per cent. of the insured population work in the salt
industry (again along the River Weaver), Middlewich, and Sandbach. On the banks
of the Mersey, and served also by the Manchester Ship Canal and by several older
but still navigable canals, are Widnes, Runcorn, and Warrington, lying mid-way
between the saltfield
1
and the Lancashire coalfield, and with waterway
connection to the textile centre of Manchester and to the port of Liverpool
(source of imported fats, vegetable oils, pyrites, and other raw materials). In
Widnes and Runcorn about 40 per cent. of the insured population are employed in
chemical industries; Warrington is more diversified, and its 6 per cent.
employed in chemicals are mainly connected with soap (though several of its
other trades are closely linked with the chemical industries—e.g. leather
tanning, paper, and cardboard). Close to these towns is St. Helens, centre of
the glass industry (see below p.
548).
Of the other areas, the most important is
Teesmouth, where the salt and anhydrite deposits in the Keuper Marl were
responsible for the development between the two world wars of the vast I.C.I.
plant at Billingham, which covers 1,000 acres, and to which was added, after the
second war, an even larger works at Wilton, to the east of Midd lesbrough, and
connected to Billingham by pipe-lines under the Tees.
At Fleetwood, the chemical industry arose from the combination of. local
brine-pumping and port facilities. On the Tyne, salt- panning was an ancient
practice; the modern chemical trades are linked with the availability of fuel
and of waste gases from coke ovens and of course with the port facilities.
The association of the West Midlands with the chemical industries arises largely
from the metallurgical trades and glass manufacture— at Oldbury and Smethwick
respectively. Important outlying centres of the chemical industries are
Avonmouth (Imperial Smelting Corporation) and Wrexham, where the great Monsanto
works has grown up (in the North Wales Coalfield “Development Area”) since the
Second World War.
The explosives industry is often located—for obvious reasons— in relatively
remote areas where wide expanses of poor land are available. The best example,
particularly since it did not, like so many others, owe its origin to either of
the two world wars, is the vast works at Ardeer, on the Ayrshire coast,
established in 1873 and later incorporated in the Nobel group. A more recent
example is near Burry Port in South Wales.
There is a close association between
non-ferrous metal smelting and refining and the chemical industries, for
chemicals are used in metal processing and numerous chemical by-products are
obtained. Thus it was Mond, of Brunner, Mond & Company, who discovered with a
collaborator a process for the extraction of pure nickel and floated the Mond
Nickel Co. whose large works are at Clydach, near Swansea (cf. p. 454). The
chemical by-products of lead and zinc smelting have already been referred to
(pp. 448,449). More recently the development of the use of the rarer metals in
the electrical industries, in alloy steel making and for lightweight alloys has
widened the range of contact between chemical and metallurgical industries, and
new developments are frequent. In fact the proc essing of these metals is for
the most part done by the chemical industry and not by firms whose main interest
is in metals. Molybd enum, zirconium, tantalum, tungsten, and titanium are
examples. Tantalum has found a use in the rayon industry: it is used for the
spinnerettes in the viscose process. Titanium, being completely non-corrosive,
whilst of high strength and low density, is being developed for use in ships and
aircraft. The oxides of some of these rare metals are used in catalytic
reactions, e.g. in the production of synthetic ammonia and in petroleum
cracking.
Although the distillation of coal on a large
scale for the production of gas seems to have been practised well before the end
of the eighteenth century, and in Germany various experimenters had known the
importance of coal gas as an illuminant, it was left to two men, Murdock of
Ayrshire, and Lebonne a Frenchman, independently to develop the commercial
production of coal gas for lighting purposes. Murdock lighted a large mill at
Manchester in 1805 with gas, and in the meantime Winzau, a German, who had
failed to arouse enthusiasm in his own country, succeeded in doing so in London,
and obtained sufficient money to carry out the lighting of the greater part of
Pall Mall (January, 1807), the first public thoroughfare in the world to be
illuminated by gas. His company received a royal charter in 1812, and later
became the Gas, Light, and Coke Company. The gas industry made steady progress.
The purification of the gas is now so good that further improvement cannot be
regarded as really necessary. But up to 1890 the lighting properties of the gas
depended upon those vapours it contained which burnt with a luminous flame; but
later it was discovered that it was better and cheaper to remove from the coal
gas these luminous gases and to rely on the heating properties of the gas to
raise metallic oxides on gas mantles to so high a temperature that they became
incandescent. So the problem became to maintain the heating properties of the
gas at a maximum and to recover from the gas the vapours which had previously
given luminosity to the flame. Gas
is
produced by heating bituminous coal in closed vessels
called retorts. The volatile gas is removed and the porous coke, which remains,
contains as high a proportion of carbon as anthracite and is used for similar
purposes, especially for central heating. Gas, by reason of its nature, must
almost of necessity be produced near centres of consumption, and the main
geographical factor arising in the location of gasworks is the ease of import of
the bulky raw material, coal. Hence the larger works, for example in London,
tend to be riverside works. In the smaller towns the gasworks are almost
invariably situated conveniently near the railway. A by-product of gas
production is coke for domestic and industrial use. The production of
metallurgical coke is a separate industry.
The aniline-dye industry originated in England
in 1856 when a brilliant young chemist, W. H. (later Sir William) Perkin, disc
overed that mauve could be manufactured from coal tar. Perkin and a band of
young German chemists working in this country developed the industry, and for
some years it flourished. But the nation did not appreciate the brilliant
discoveries, and the whole industry practically disappeared from Britain and was
built up on a commercial scale in Germany. There it became one of enormous
importance, and the artificial dyes made from coal tar practically eliminated
from the world’s markets natural dyes such as indigo.
|
Industry |
Employment |
Chief Regions
1 |
|
|
General Chemicals . . . |
100 |
N.W. 31; N. 21; London and |
|
|
. |
S.E.
15. |
||
|
Explosives and fireworks . |
25 |
Scot. 9; Wales 4; N.W. 4. |
7; |
|
Toilet preps. and perfumery Fertilisers and
insecticides |
9 |
London and S.E. 7. Scot. 3; London and S.E. |
2; |
|
Paint and varnish . . . |
33 |
Yorks, 2; N. 2. London and S.E. 12; N.W. |
5; |
The outbreak of war in 1914 found this country almost
totally unprepared so far as a supply of dyestuffs was concerned. But two firms
were still in existence and they were galvanised into immediate action; for it
has been truthfully said that without dyestuffs an army cannot be uniformly
clothed, and an army without a uniform is but a rabble. A new firm started work
at Manchester and Scottish Dyes, Ltd., at Grangemouth in Scotland. Amazing prog
ress was made, so that by 1918 it was not surprising to find an amalgamation of
interests under the name of British Dyestuffs Corporation, Ltd., which later
purchased a controlling interest in Scottish Dyes. In turn, as part of the
chemical industry, this became an essential constituent of Imperial Chemical
Industries, Ltd., and British dyestuffs can claim to be unrivalled in the world.
About 80 per cent. of dyes used in Britain are now home-produced, and a
considerable proportion of the output emanates from the textile regions of
Lancashire and Yorkshire, where Manchester and Huddersfield are the principal
centres.
Intimately linked with the dyestuffs industry, since similar comp licated
organic molecular structures are involved, and similar raw materials, are the
manufactures of pharmaceuticals, insecticides, and other agricultural chemicals,
rubber additives, and plasticisers for plastics.
There is an unexpectedly close connection
between explosives and artificial silk, both being closely connected with
cellulose, which is the main constituent of dry vegetable matter, and thus forms
the bulk of wood, cotton, linen, and many other vegetable products. Thus, if
cotton rags are treated with caustic soda, washed, bleached, and dried the
production of cellulose is the result. If the cellulose is dissolved in a
mixture of sulphuric and nitric acid nitro-celluloses are formed, which are in
general highly inflammable or explosive substances. Gun cotton is a
nitro-cellulose, and cordite and other explosives are closely associated. On the
other hand harmless solids can be formed from similar bases. Examples are
xylonite—a mixture of cellulose-nitrate and camphor with a little castor oil,
and celluloid which is a mixture of cellulose-nitrate and camphor. The principle
of making artificial silk is to dissolve cellulose-nitrate in a suitable organic
solvent, and then to squeeze the liquid through very tiny holes so as to make a
thread which, when it is dry, is as fine or almost as fine as the silken thread
naturally spun by the silkw orm. As early as 1885 a few articles were produced
under the description of artificial silk; but the real progress of the industry
is very largely post-1918. In the production of viscose silk, wood-pulp or some
other material containing cellulose is treated with caustic soda and then with
carbon bisuiphide; a yellow viscous liquid being obtained which when put into a
suitable precipitating bath yields tough fibres. A further process is used to
remove the sulphur from such fibres. Another variety of artificial silk is
acetate silk, prod uced by treating cellulose with acetic acid (derived from
petroleum refining). Another artificial fibre is alginate yarn, a soluble yarn
of great value in the production of certain types of textile fabrics, since it
disappears when the fabric is washed; it is made from seaweed. The recent
development of “nylon” and “terylene” is intimately bound up with the great
post-war expansion of the petrochemicals industry (cf. p. 566), for these new
fibres are largely made from petroleum derivatives.
Although the making of soap is essentially a
chemical industry, soap making long antedates the development of the chemical
industry properly speaking. The art of boiling together oils and fats with an
alkali (which was usually obtained by burning seaweed, thus giving an impure
alkali containing both soda and potash), and then adding common salt which
throws out the soap as a curd that can be cut into bars, tablets, and the like
was practised long ago. But clearly the modern connection with the chemical
industry is the large requirement of alkali and salt. In the early days almost
any fat or oil was used, and it is not surprising therefore to find the early
soap-boiling works scattered in different places throughout the country. The
great business of Lever Brothers was built up very largely on the utilisation of
vegetable oils instead of animal fats as the basis for soap making. The greatest
of all the centres created by the company, Port Sunlight, was initiated in 1888.
W. H. Lever selected this site because “it was a rural area where ample acreage
could be secured adjacent to both rail and water transport with reasonable
facilities for obtaining the necessary supply of labour.” Incidentally the area
chosen is conveniently situated to the alkali-manufacturing centres of the
Mersey. The Lever interests gradually absorbed many of the soap- making
companies; there was close connection also with those who were using oil for
other purposes, such as the British Oil & Cake Mills Ltd., and finally, in 1929,
came the merging of interests with the Margarine Union, which had been using
vegetable oil for the manufacture of margarine. The whole great combine is known
as Unilever Limited, which manufactures in fifty countries and employs more than
a quarter of a million people.
Although Port Sunlight dominates the industry, there are other centres in the
North-Western Region, notably Warrington; outside Merseyside, the port area of
London, and several centres in Scotland such as Renfrew, Paisley, and
Grangemouth are concerned with soap manufacture.
The soap industry is turning in part to new
products—synthetic detergents or “syndets,” which are now flooding the market
under numerous proprietary names. These detergents—which started by being soap
substitutes and have turned out to be in fact very much better than soap for
many purposes, particularly with hard water— are made for the most part from oil
refinery waste products, and both the soap firms and the petrochemical interests
are involved.
The manufacture of glass is essentially a
chemical process. All types of glass are made by heating in a furnace, silica
(which is usually obtained in the form of sand) with soda and lime and the
oxides of other metals such as magnesium, aluminium, boron or lead, according to
the type of glass required—e.g. a large proportion of borax is used for “pyrex”
heat-resisting glass. The mixtures are very complex; ordinary bottle glass
contains no less than twelve chemical elements. Plate glass is rolled in
continuous sheets while soft; glass tubing is drawn out; glass bottles are cast
in moulds or are blown. The main requirements of the glass industry are thus a
suitable supply of fuel, sand, and chemicals, chiefly soda, lime, magnesia, and
borax from the heavy chemical industry. For the finer types of glass, including
colourless milk bottles, the purest white sand, free from coloured impurities,
must be used. Such sand comes from the superficial Shirdley Hill Sands of
South-West Lancashire and from occasional pockets in the Lower Greensand
formation—as at King’s Lynn, Leighton Buzzard, and Redhill—or is imported from
Belgium or elsewhere. A new source of high- quality sand was discovered during
the Second World War at Lochaline in western Scotland. For coloured bottles,
less-pure raw material is needed, and several other geological formations, as
well as those named above, contribute supplies.
The glass industry in 1948 gave employment to over 60,000 people, of whom 24,000
were concerned with bottle-making. The London area, by reason of its huge market
for milk and beer bottles, and easy access to Lower Greensand and imported sand
supplies, has a very large bottle-making industry, and other centres are
Doncaster and St. Helens. The latter town is also the chief in the country for
glass- making other than bottles. The geographical influences here were simple—Shirdley
Hill sands and coal almost on the spot and the great mid-Mersey chemical
industry only a few miles away. Interesti ng, if less obvious, locations are
Stourbridge, on the edge of the Black Country (where the glass industry was
started by foreign refugees several hundred years ago), Smethwick, chief British
centre for the making of lighthouse lanterns, and Sunderland, home of “pyrex”
glass.
The plastics industry is essentially a product of the last two or three decades; of small importance before about 1928, it was greatly stimulated by the rapid growth of the electrical and radio industries, and great advances were made during the Second World War. The output increased more than four-fold during the decade
The plastics industry, like that of artificial
fibres, consists of two parts, the actual production of the synthetic resins and
moulding powders, which is very definitely a chemical industry, and the
manipulation of the materials, which are now used in a great variety of
industries, electrical, radio, furniture and household fittings, aircraft,
boat-building, paint, plastic crockery, glass substitutes, brushes, raincoats
and table-covers, and an ever-increasing range of miscellaneous manufactures.
But there is a much closer link between the two parts than in the case of the
textile fibres. The basic chemicals, which are derived from salt, coal, oil,
nitrogen, etc., are married together by the process known as polymerisation into
plastic materials. There are two major types: (i) thermos etting plastics, which
harden by chemical action brought about by heating and cannot subsequently be
softened again; they are moulded by compression; (ii) thermoplastics, which are
moulded by injection or are produced in flexible sheets, rods, or tubes, in a
wide range of colours. The thermo-setting plastics are based on phenol (derived
from coal tar), formaldehyde (from carbon monoxide and hydrogen) and urea (from
ammonia and carbon dioxide); they are used for such things as door knobs,
laminated sheets, plastic crockery, electrical insulators, table-tops, etc., in
paint manufacture and as a bonding material in plywood and furniture. The
thermoplastics are of two kinds, those derived from cellulose, the manufacture
of which depends on imported cotton linters, and the “synthetic” materials,
derived mainly from coal and petroleum but using also salt and limestone. The
synthetic plastics are of quite recent development, mainly during and since the
Second World War, and include polythene (based on ethylene derived from oil
cracking), “perspex” or glass-substitute (derived from acetone),
polyvinyichioride
(“
P.V.C.”—based on carbide and chlorine), much
in use for raincoats, curtains, table-covers, handbags, wall and floor
coverings, etc., nylon (from benzene), used as a textile and for brush-bristles,
rope, racket strings, and as sheet and film for packagi ng, and polystyrene
(from benzene and ethylene), used for toys, table-ware, toilet articles, radio
parts, etc.
The wide variety of raw materials, both
home-produced (coal erivatives, salt, limestone) and imported (petroleum,
refined at the ports of entry—see p. 567—carbide, cotton linters, wood pulp,
resins, etc.) and the equally wide variety of products and markets result in a
widespread distribution of the industry. Moreover, the industry has been taken
up by many firms previously engaged in other or allied trades, and so it is not
easy to generalise about location factors. But since there is little wastage of
raw materials during manufacture, labour supply and markets rather than raw
materials tend to be of most significance—hence the importance of the Greater
London and Birmingham areas, for example—whilst the “light” and largely
“footloose” character of the manipulation side of the industry has made it a
suitable one for setting up in the Development Areas. The list of localities
contains more une xpected and not readily explicable places than is the case
with almost any other manufacturing industry. The I.C.I. Works at Northwich
produces most of the known varieties, and its new plant at Wilton, near
Middlesbrough (see p. 543) will produce “terylene”; outside the main “chemical”
areas the chief I.C.I. establishment is at Welwyn Garden City.
There are many other chemical industries which
deserve more than passing mention. The most important is the
pharmaceuticals
branch, which in fact can be divided into six quite
distinct subd ivisions—(i) medical fine chemicals (e.g. aspirin), (ii)
antibiotics (e.g. penicillin)—largely a recent offshoot from brewing and dist
illing, (iii) potent active principles from vegetable and animal sources (e.g.
from opium and from liver), (iv) vaccines and sera, (v) “galenicals” (extracts,
syrups, ointments), (vi) compounded and packaged preparations. This is an
industry which has expanded enormously during the last few decades; indeed
probably three- quarters of the drugs and medicines now on the market were
unknown twenty years ago. It is also the largest employer of labour
—much ot it highly skilled and much of it female—of all the chemical industries
outside the general heavy chemical group. A large prop ortion of the industry is
in the Metropolitan area—with important establishments in the newer industrial
localities such as Dagenham and Welwyn Garden City; in the provinces the
Liverpool and Manchester areas are important and, of course, Nottingham, the
home of Boots.
Fertilisers,
together with insecticides and sheep-dips,
make another important branch. Dependent partly on imported raw materials
(phosphates) and partly on home-produced chemicals (e.g. sulphate of ammonia),
and having a widespread agricultural market, this industry is fairly well
scattered, with an emphasis on port locations and centres (e.g. Ely) within the
main agricultural areas. It is also increasingly bound up with petrochemicals,
and some oil refining concerns are now in the business.
Paint and varnish
manufacture, depending on pigments, solvents,
oils, and resins, is a large employer of labour in several of the major
industrial districts, particularly Greater London, South Lancashire, and West
Yorkshire; in Glasgow and on Tyneside it is clearly linked with the
ship-building industry. It is sometimes also allied to the manufacture of edible
oils and of oilskin garments, and to an increasing extent it is drawing on the
products of the plastic industry. Another closely connected trade is that of
oil-cloth
and
linoleum
manufacture. Oxidised linseed oil and cork are mixed
with gum or resin and with pigments and pressed out on a rough canvas body (made
from jute) between steam-heated rollers. Kirkcaldy (Fifeshire) has remained the
main seat of manufacture in Britain, with an offshoot at Dundee; the link with
the jute industry is clear